SIGA Technologies has announced that the Canadian Department of National Defence (CDND) has signed a contract with Meridian Medical Technologies to purchase oral TPOXX (tecovirimat).
The move comes after SIGA signed an international promotion agreement with Meridian under which, the latter will promote the sale of the company’s oral TPOXX to treat smallpox in all markets, except for the US and South Korea.
Under the latest $14.3m contract, CDND will purchase up to 15,325 courses of oral TPOXX over four years. The contract will have an initial purchase of 2,500 courses for $2.3m.
Oral TPOXX has been developed through funding and collaboration with the Biomedical Advanced Research and Development Authority at the US Department of Health and Human Services.
SIGA Technologies CEO Dr Phil Gomez said: “The current Covid-19 pandemic has reminded us all that preparedness with medical countermeasures is critical for responding effectively to any infectious disease outbreak.
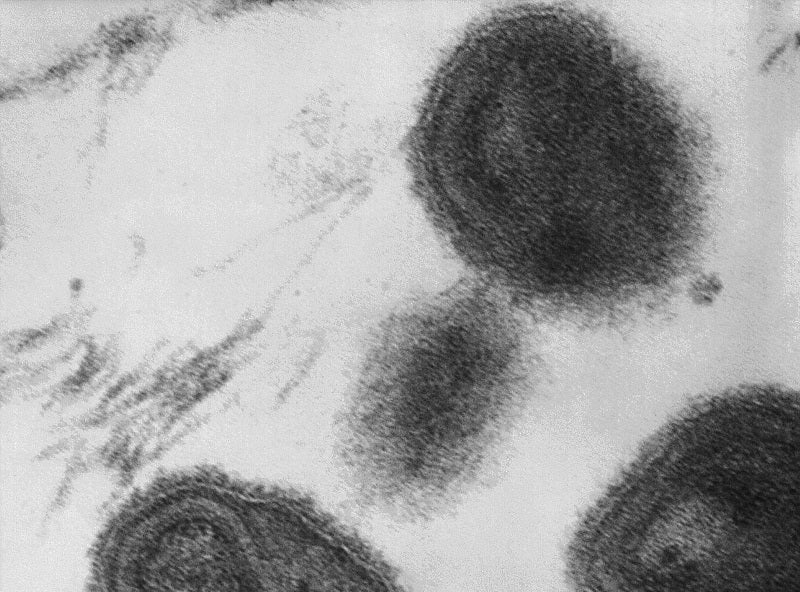
“Smallpox remains a key threat, and TPOXX is an important component of any smallpox response plan.
“This procurement by the Canadian military is an important first step, and we look forward to continuing to work with Meridian to support potential future procurements with both the CDND and Health Canada to protect Canada’s military and civilian population.”
Under the Canadian contract, following the regulatory approval in Canada, SIGA expects delivery of additional 12,825 courses.
Previously, SIGA secured a contract from the CDND to fund this regulatory submission.
The Canadian regulatory filing is targeted to be completed by the second half of this year, with regulatory approval expected next year.
SIGA is responsible for the manufacture and delivery of product, whereas Meridian is the counterparty to the Canadian contract.
On 13 July 2018, the oral TPOXX was approved for the treatment of smallpox to mitigate the impact of a potential outbreak or bioterror attack, by Food and Drug Administration (FDA).



